FDA approves ReWalk robotic exoskeleton (Video)
Posted by: Jon Ben-Mayor on 06/29/2014 06:40 AM
[
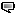 Comments
]
Comments
]
The ReWalk device was created in 2011 by Israeli company Argo Medical Technologies’ founder Dr. Amit Goffer, who in 1997 was in a car accident that left him a quadriplegic - an incident that was understandably a driving force for this project. This device will dramatically change the lives of many people suffering the same lack of mobility.
According to the press release, Exoskeleton leader ReWalk Robotics announced today that the U.S. Food and Drug Administration has cleared the company’s ReWalk Personal System for use at home and in the community. ReWalk is a wearable robotic exoskeleton that provides powered hip and knee motion to enable individuals with Spinal Cord Injury (SCI) to stand upright and walk. ReWalk, the only exoskeleton with FDA clearance via clinical studies and extensive performance testing for personal use, is now available throughout the United States.
The FDA has issued a marketing clearance for the ReWalk, which was granted via a rigorous de novo process that involved multiple clinical studies demonstrating safety and effectiveness of the technology.
“This revolutionary product will have an immediate, life-changing impact on individuals with spinal cord injuries,” said Larry Jasinski, CEO of ReWalk Robotics. “For the first time individuals with paraplegia will be able to take home this exoskeleton technology, use it every day and maximize on the physiological and psychological benefits we have observed in clinical trials,” he added. “This is truly the beginning of ‘ReWalking’ as a daily reality in the U.S.”
Derek Herrera, a Captain in the U.S. Marine Corps, is a paraplegic trained on the ReWalk Personal System, and will be one of the first Americans to own the ReWalk. “I see this as a milestone for people in my same situation who will now have access to this technology – to experience walking again, and all of the health benefits that come with ReWalking,” Captain Herrera said. The Marine Special Operations Command Foundation (MARSOC Foundation) will be donating the funds for Herrera’s ReWalk system; Herrera works for the Marine Special Operations Command. “It will be incredible for me to regain independence, to use the system to walk and stand on my own,” he added.
ReWalk provides user-initiated mobility through the integration of a wearable brace support, a computer-based control system and motion sensors. The system allows independent, controlled walking while mimicking the natural gait patterns of the legs, similar to that of an able-bodied person. In addition to the ability to stand and walk independently, clinical studies of the ReWalk Rehabilitation system show significant health benefits to the user, on both a physiological and psychological level.
ReWalk has been tested extensively in the U.S., Europe, and Israel. The ReWalk system is supported by the most published data of all exoskeleton systems in the rehabilitation market, and is used by more people worldwide than all other exoskeleton systems combined.
The ReWalk Personal System is available to consumers upon meeting requirements from a medical examination and successful completion of the required training program. The company also offers a comprehensive maintenance and warranty program for the system. Each systems costs around $72,000, which in the scheme of things is not a bad trade for regained mobility.
According to the press release, Exoskeleton leader ReWalk Robotics announced today that the U.S. Food and Drug Administration has cleared the company’s ReWalk Personal System for use at home and in the community. ReWalk is a wearable robotic exoskeleton that provides powered hip and knee motion to enable individuals with Spinal Cord Injury (SCI) to stand upright and walk. ReWalk, the only exoskeleton with FDA clearance via clinical studies and extensive performance testing for personal use, is now available throughout the United States.
The FDA has issued a marketing clearance for the ReWalk, which was granted via a rigorous de novo process that involved multiple clinical studies demonstrating safety and effectiveness of the technology.
“This revolutionary product will have an immediate, life-changing impact on individuals with spinal cord injuries,” said Larry Jasinski, CEO of ReWalk Robotics. “For the first time individuals with paraplegia will be able to take home this exoskeleton technology, use it every day and maximize on the physiological and psychological benefits we have observed in clinical trials,” he added. “This is truly the beginning of ‘ReWalking’ as a daily reality in the U.S.”
Derek Herrera, a Captain in the U.S. Marine Corps, is a paraplegic trained on the ReWalk Personal System, and will be one of the first Americans to own the ReWalk. “I see this as a milestone for people in my same situation who will now have access to this technology – to experience walking again, and all of the health benefits that come with ReWalking,” Captain Herrera said. The Marine Special Operations Command Foundation (MARSOC Foundation) will be donating the funds for Herrera’s ReWalk system; Herrera works for the Marine Special Operations Command. “It will be incredible for me to regain independence, to use the system to walk and stand on my own,” he added.
ReWalk provides user-initiated mobility through the integration of a wearable brace support, a computer-based control system and motion sensors. The system allows independent, controlled walking while mimicking the natural gait patterns of the legs, similar to that of an able-bodied person. In addition to the ability to stand and walk independently, clinical studies of the ReWalk Rehabilitation system show significant health benefits to the user, on both a physiological and psychological level.
ReWalk has been tested extensively in the U.S., Europe, and Israel. The ReWalk system is supported by the most published data of all exoskeleton systems in the rehabilitation market, and is used by more people worldwide than all other exoskeleton systems combined.
The ReWalk Personal System is available to consumers upon meeting requirements from a medical examination and successful completion of the required training program. The company also offers a comprehensive maintenance and warranty program for the system. Each systems costs around $72,000, which in the scheme of things is not a bad trade for regained mobility.
Comments






